I. ESSENTIAL ELEMENTS
|
|
|
to Plants |
|
dry tissue |
| Macronutrients | ||||
| Nitrogen | N | NO3-, NH4+ | 14.0 | 4.0% |
| Phosphorus | P | PO4-3, HPO4-2, H2PO4- | 31.0 | 0.5% |
| Potassium | K | K+ | 39.1 | 4.0% |
| Magnesium | Mg | Mg2+ | 24.3 | 0.5% |
| Sulfur | S | SO42- | 32.1 | 0.5% |
| Calcium | Ca | Ca2+ | 40.0 | 1.0% |
| Micronutrients | ||||
| Iron | Fe | Fe2+ ,Fe3+ | 55.9 | 200ppm |
| Manganese | Mn | Mn2+ | 54.9 | 200ppm |
| Zinc | Zn | Zn2+ | 65.4 | 30ppm |
| Copper | Cu | Cu2+ | 63.5 | 10ppm |
| Boron | B | BO32- , B4O72- | 10.8 | 60ppm |
| Molybdenum | Mo | MoO42- | 96.0 | 2ppm |
| Chloride | Cl | Cl- | 35.5 | 3000ppm |
| Essential but not applied | ||||
| Carbon | C | CO2 | 12.0 | 40% |
| Hydrogen | H | H2O | 1.0 | 6% |
| Oxygen | O | O2, H2O | 16.0 | 40% |
II. MACRONUTRIENTS
A. Nitrogen (N)
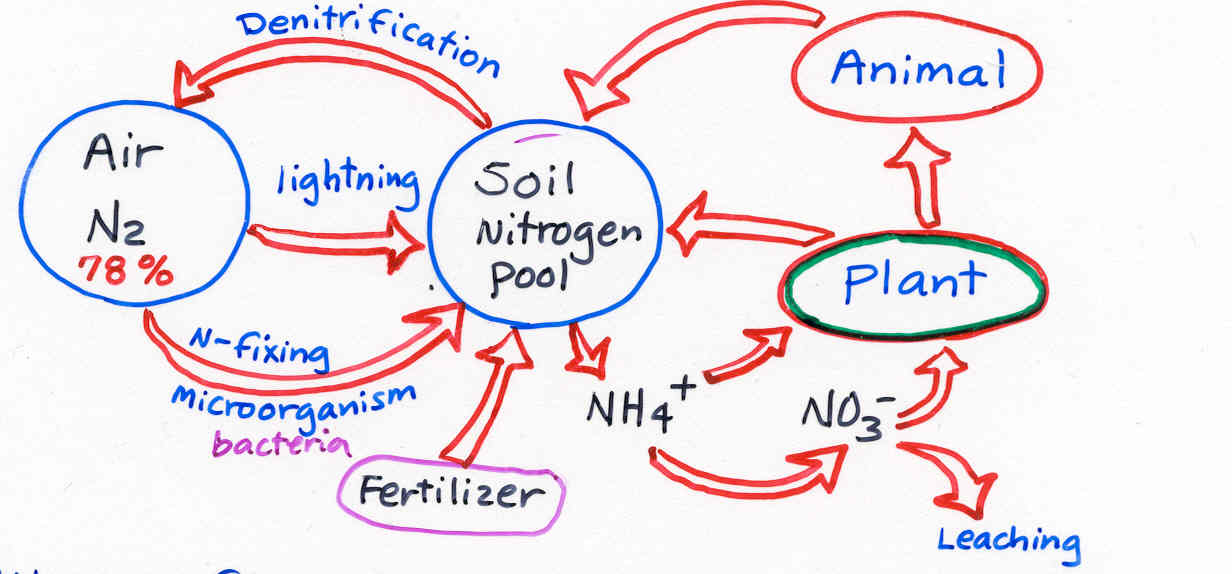
1) The Soil Nitrogen Cycle

Nitrogen fixation - Transformation
of atmospheric N to nitrogen forms available to plants
N-fixing bacteria - Rhizobium
(symbiotic) in legumes (soybean, peas,
honeylocust; species in Farbaceae)
Azotobacter (non-symbiotic)
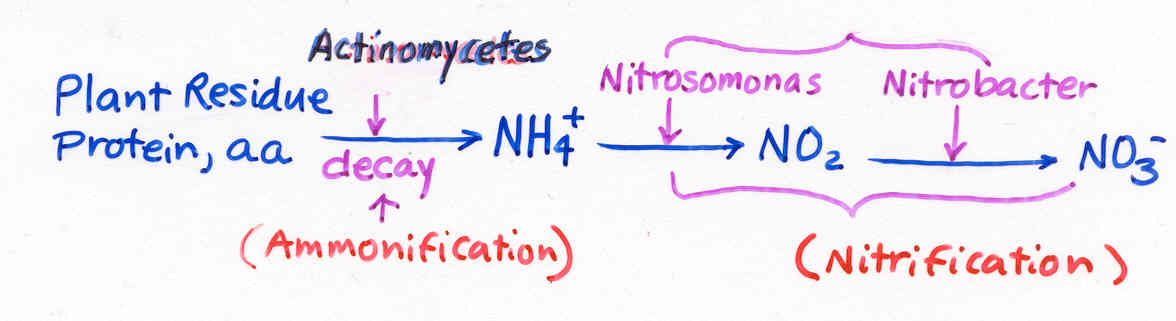
Ammonification and Nitrification
2)
N Functions in Plants
- component of proteins, enzymes, amino acids, nucleic acids, chlorophyll
- C/N ratio (Carbohydrate : Nitrogen ratio)
High C/N ratio-----Reproductive
Low C/N ratio-----Vegetative
- Transamination
NO3 >NH2
>Glutamic Acid (a.a)
> other a.a. >Protein,
Enzyme
- Essential for fast growth, green color
3)
Deficiency and Toxicity Symptoms
Deficiency: Reduced growth
Yellowing of old leaves
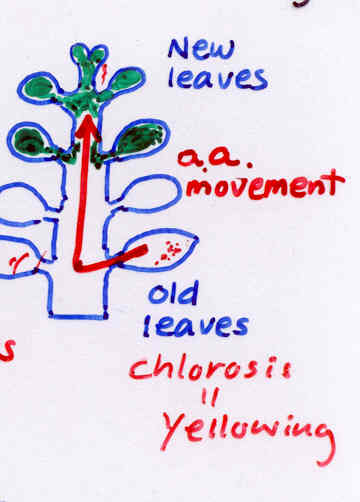
Protein degradation >amino
acids >move to new
leaves
(old leaf)
4) Fertilizers

-Ammonium Nitrate (NH4NO3) (cheap)
Calcium Nitrate (CaNO3) (expensive)
Potassium Nitrate (KNO3) (expensive)
Urea (CO(NH2)2) (cheap)
-Most plants prefer 50 : 50
NH4+ : NO3-
2:5
NH4+ form of Nitrogen ------- Soil pH lowers
(good for blueberry, Azalea)
NO3- form of Nitrogen ------- Soil pH increases
-Organic fertilizer (manure, plant residue)- slow acting
-Can be applied foliarly
B. Phosphorus
(P)
1) Soil Relations
-Mineral apatite (Ca5F(PO4)3) provides
P
-Relatively stable in soil
-Has a low mobility (1-2 cm) (therefore top dressing not effective)
2)
Plant Funtions
-component of nucleic acids (DNA, RNA), phospholipids, coenzymes, high-energy
phosphate bonds (ADP,ATP)
-seeds are high in P
3)
Deficiency and Toxicity
-P is mobile in plant tissure (deficiency occurs in older leaves)
-Deficiency------dark, purplish color on older leaves
-Exess P--------causes deficiency symptoms of An, Cu, Fe, Mn
4)
Fertilizers
-Superphosphates (May contain F)
Single Superphosphate (8.6% P) CaH4(PO4)2
Triple Superphospate (20% P)
-Ammonium Phosphate ((NH4)2PO4)
NH4HPO4
phosphoric acid (H3PO4) potassium phosphate, ect
-Bonemeal
-Absorption of available forms is influenced by pH
C. Potassium
(K)
1) Soil Relations
-Present in large amounts in mineral soil
-Low in organic soil
2)Plant
Functions
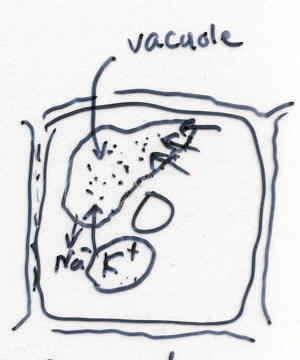
-Activator of many enzymes
-Regulation of water movement across membranes and through stomata (Guard
cell function)
3)
Deficiency and Toxicity Symptoms
-Deficiency----- marginal necrosis, browning on leafs, older leaves more
affected
-Toxicity------- leaf tip and marginal necrosis
4)Fertilizers
-Potassium cloride (murate of potash) KCl
-Potassium sulfate K2SO4
-Potassium nitrate KNO3
D. Calcium
(Ca)
1) Soil Relations
-present in large quantities in earth's surface (~ 4%)
1% in U.S. top soils
-influences availability of other ions from soil
2) Plant Functions
-Component of cell walls
-Involved in membrane function
-Calcium pectate in middle lamela
Calcium pectate is immobile in tissues
3)
Deficiency and Toxicity
-Deficiency symptoms in young leaves and new shoots (immobile)
stunted growth, leaf distortion, necrotic spots, shoot tip death, blossom-end
rot in tomato

4)
Fertilizers
-Agricultural meal (finely ground CaCO3. MgCO3
)
-Lime (CaCO3) gypsum (CaSo4)
-Superphosphate
Bonemeal- organic
E. Sulfur
(S)
1) Soil Relations
-present in mineral pyrite (FeS2 fool's gold), sulfides (S-mineral
complex) and suldates (involving SO4-2)
-mostly contained in organic matter
-acid rain provided sulfur
2)
Plant Funtions
-component of amino acids (Methionine cysteine)
-constituent of coenzymes and vitamins
-responsible for pungency and flavor (Onion, garlic, mustard)
3)
Deficiency and Toxicity
-Deficiency---------------- light green or yellowing on new growth (Sulfur
is immobile in tissues)
-Toxicity------------------ Not commonly seen
4)
Fertilizers
-Gypsum (CaSO4)
-Magnesium Sulfate (MgSO4)
-Ammonium Sulfate ((NH4)2SO4)
-Elemental Sulfur
F. Magnesium
1) Soil Relations
-presentin soil as an exchangeable cation (Mg2+)
-similar to Ca2+ as a cation
2)
Plant Functions
-core component of chlorophyll molecule
-catalyst for certain enzyme activity

3)
Deficiency and Toxicity
-Deficiency------------------ Interveinal chlorosis on mature leaves
(Mg is highly mobile in plant)
-Toxicity--------------------- Causes deficiency symptomsof Ca, K
4)
Fertilizers
-Dolomite ( CaCO3 . MgCO3 mixture)
-Epsom salt (MgSO4)
-Magnesium Nitrate (Mg(NO3)2)
III. MACRONUTRIENTS
A. Iron (Fe)
Component of cytochromes- for photosynthesis
Essential for nitrogen fixation (Nitrate reductase) and respiration
Deficiency------
Interveinal chlorosis on new growth
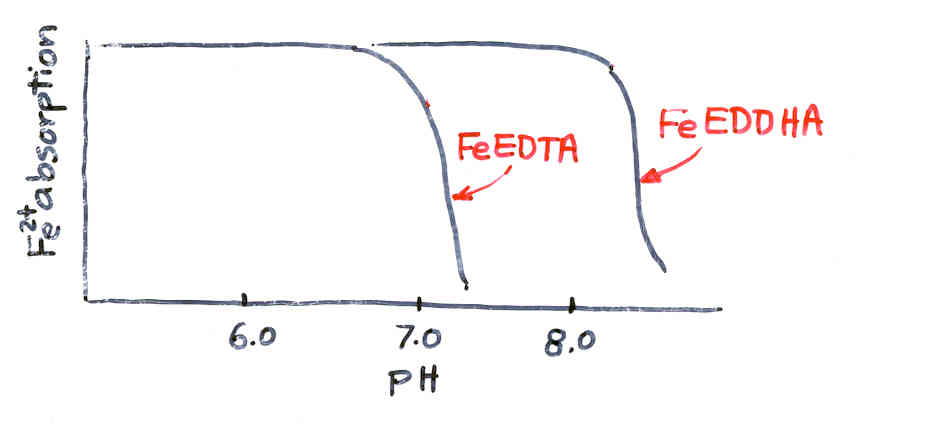
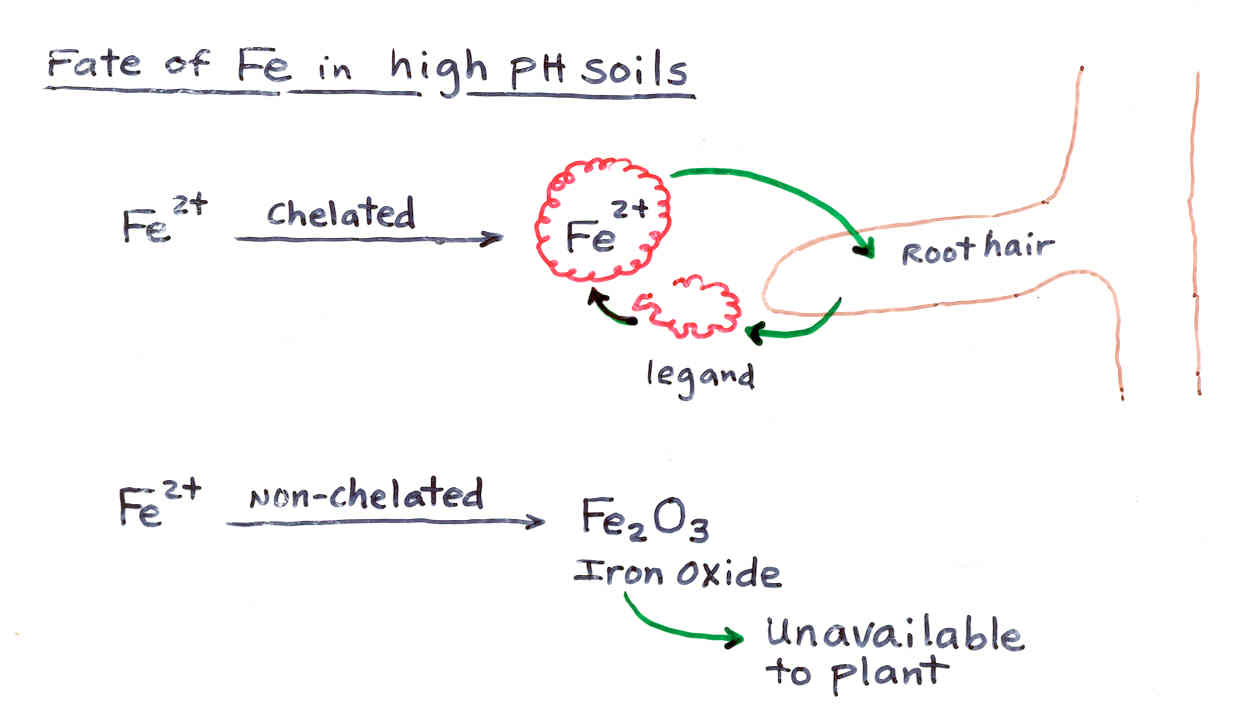
Iron Chlorosis at High pH
Remedy- Use Iron chelates
lower soil pH
FeEDTA (Fe330)- Stable at pH <7.0
FeEDDHA (Fe138)-Stable even when pH>7.0
EDTA- Ethylene diamine Tetraacetic acid
EDDHA- Ethylene diamine dihyroxy phenylacetic acid
B. Manganese
(Mn)
Required for chlorophyll synthesis; Oxygen evolution in PHS; activates
some enzyme systems
Deficiency-----
Mottled chlorosis between main veins of new leaves (Mn is imobile) Similar
to iron chlorosis
Toxicity------- Chlorosis on new growth with small, numerous dark spots
Deficiency occurs in high pH soils; toxicity at low pH
Fertilizers-----
MnSO4
MnEDTA (chelate) for high pH soils
C. Boron (B)
Involved in Carbohydrate metabolism
Essential for flowering, pollen germination, nitrogen metabolism
Defficiency--
New growth malformed (distorted), flowering and fruit set depressed, roots
and tubers cracked
Toxicity----- Twig dieback, fruit splitting, leaf edge burns
Fertilizers---
Boraz (Na2B4O7 . 10H2O)
Calcium borate (CaB4O7 . 4H2O)
D. Zinc (Zn)
Involved in protein synthesis, IAA (natural axin) synthesis
Deficiency (in calcarious soil adn high pH)
-Growth suppression, reduced internode length
-Rosetting, interveinal chlorosis on young leaves
(Zn is immbile in tissues)
Toxicity (at low pH)
-Growth reduction, leaf chlorosis
E. Molybdenum
(Mo)
Required for nitrate reductase activity, vitamin synthesis
Root-nodule bacteria also requires Mo
Defficiency
(at low pH)
-pale-green cupped leaves (young leaves-immobile)
-'strap' leaf in broad leaf plants
Toxicity
-Chlorosis with orange color pigmentation
Fertilizer- Sodium Molybdate
F. Copper
(Cu)
-Essential component of several enzymes for chlorophyll synthesis, carbohydrate
metabolism.
Defficiency---varied
symptoms
rosette or "witch's" broom
Toxicity------ Chlorosis
Fertilizer--- Copper Sulfate (CuSO4)
G. Chlorine
(Cl)
-Essential for photosynthetic ozygen evolution
Defficiency-- Normally not existing but can be experimentally induced)
Toxicity----- Never used (Cl in ubiquitous!!!)
IV. FERTILIZER CONENTRATION CALCULATIONS
A. Analysis
1) Commercial Analysis
Based on % total weights of nitrogen (N), phosphoric oxide (P2O5)
ant potash (K2O) in that order
Ex. 10-10-10 commercial analysis contains 10% N, 10% phosphoric acid,
10% potash
2)
Elemental Analysis
Bases on % total weights of nitrogen (N), phosphorus (P), and potassium
(K), in elemental forms, arranged in that order
Ex. 10-10-10 elemental analysis contains 10% N, 10% P, 10% K
B. Conversion
of Commercial Analysis to Elemental Analysis
|
|
|
|
|
|
|
|
|
|
|
|
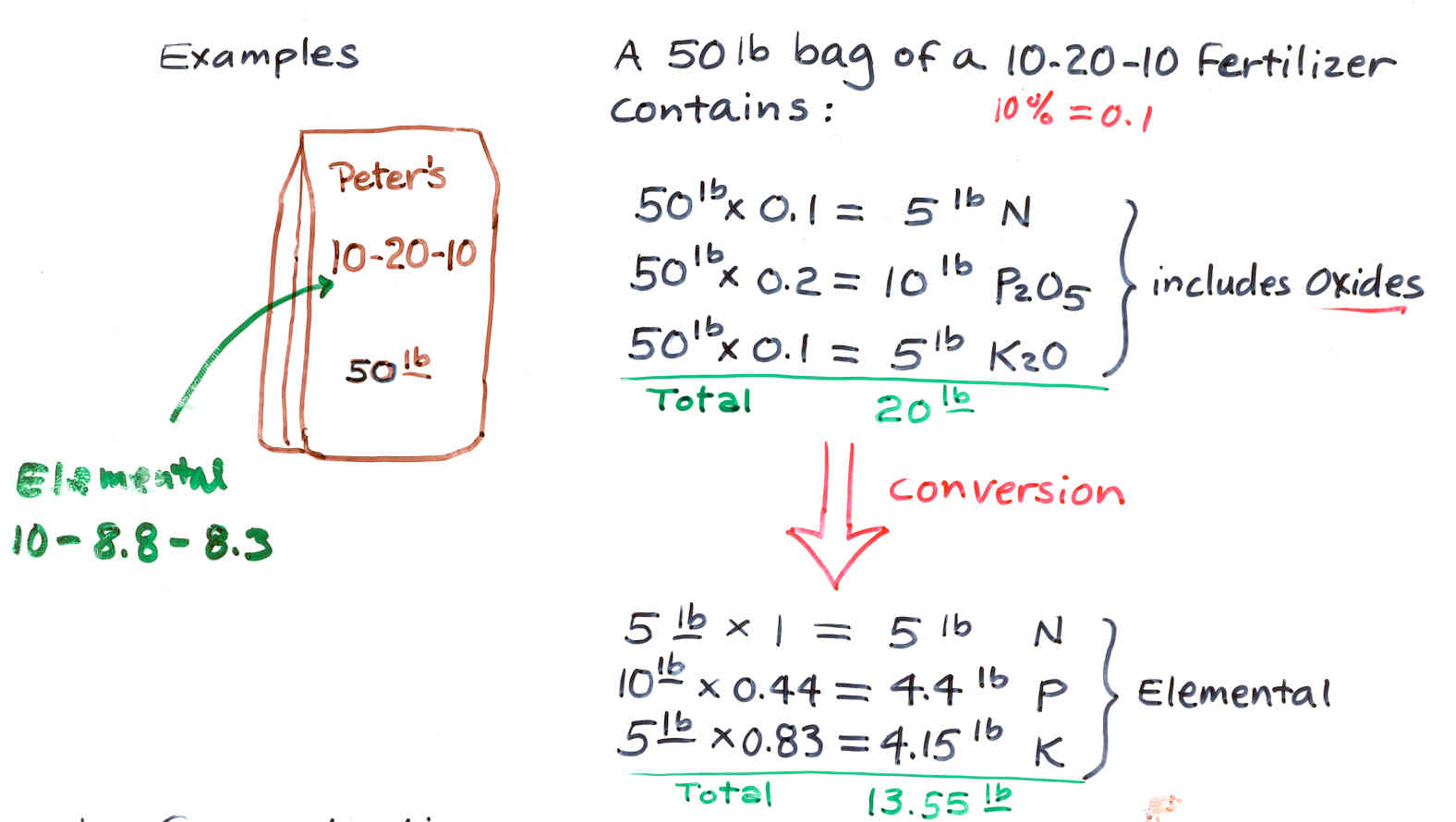
C. Concentrations
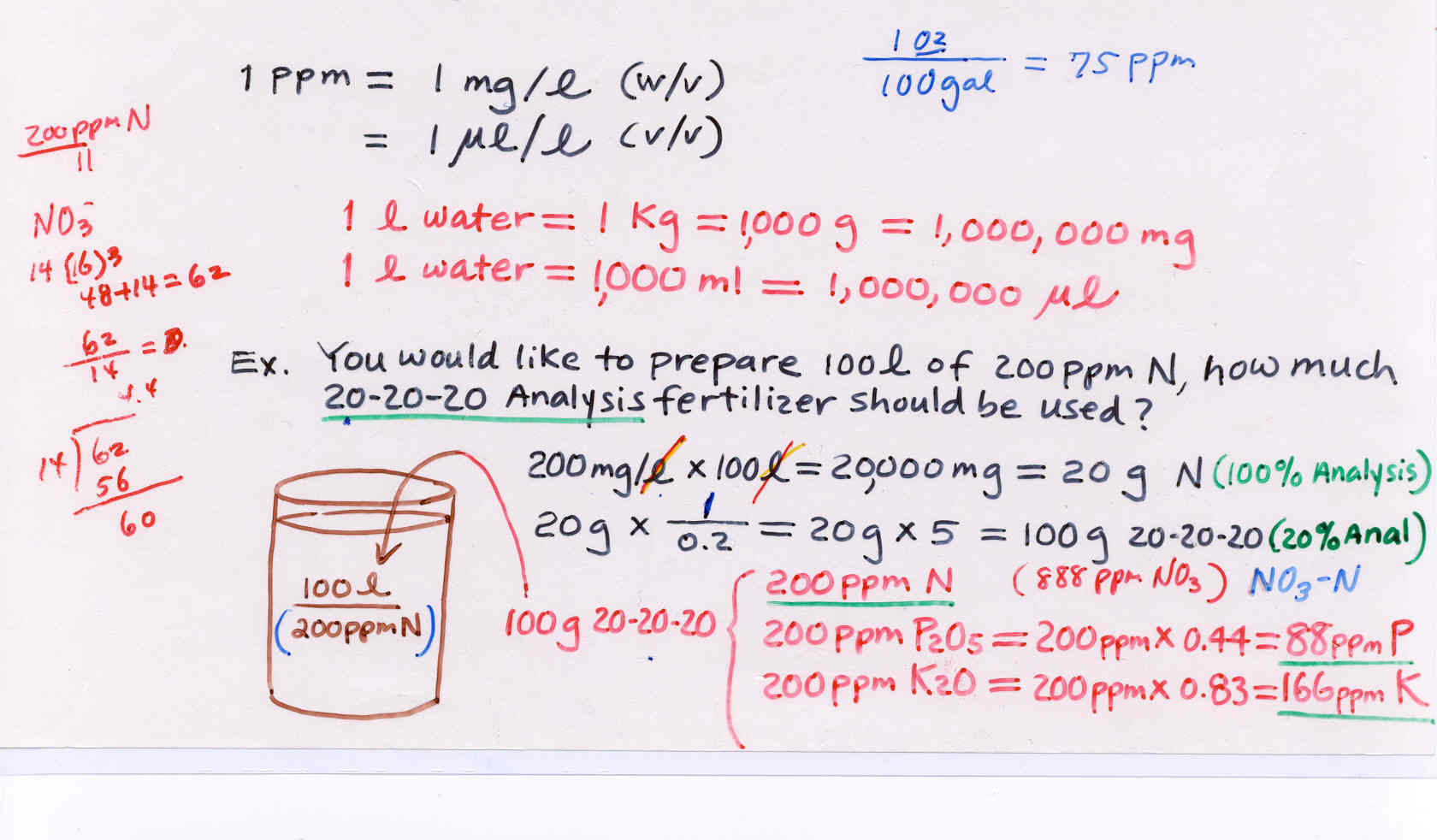
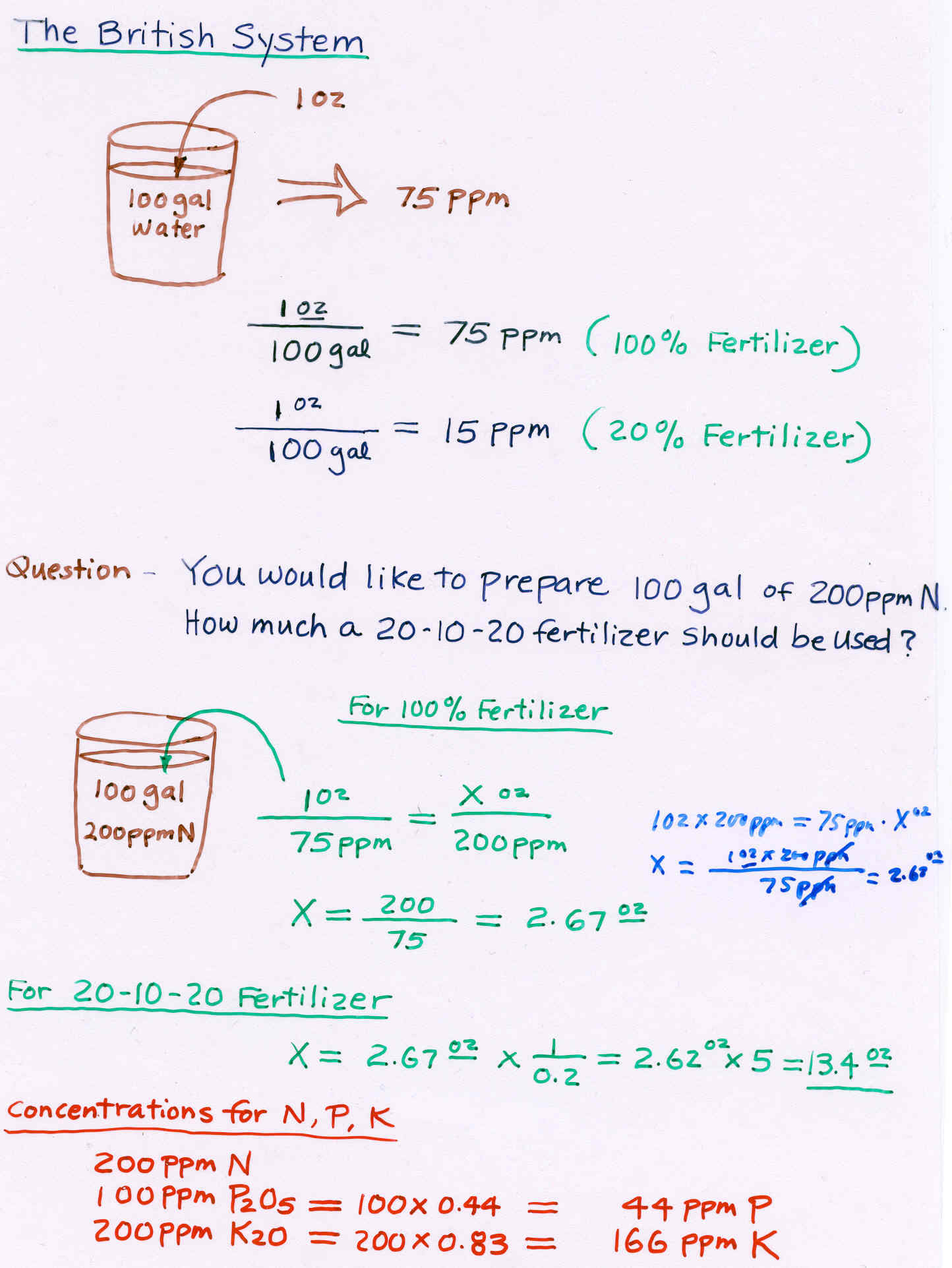
1. Units
ppm = parts per million (mg/liter)
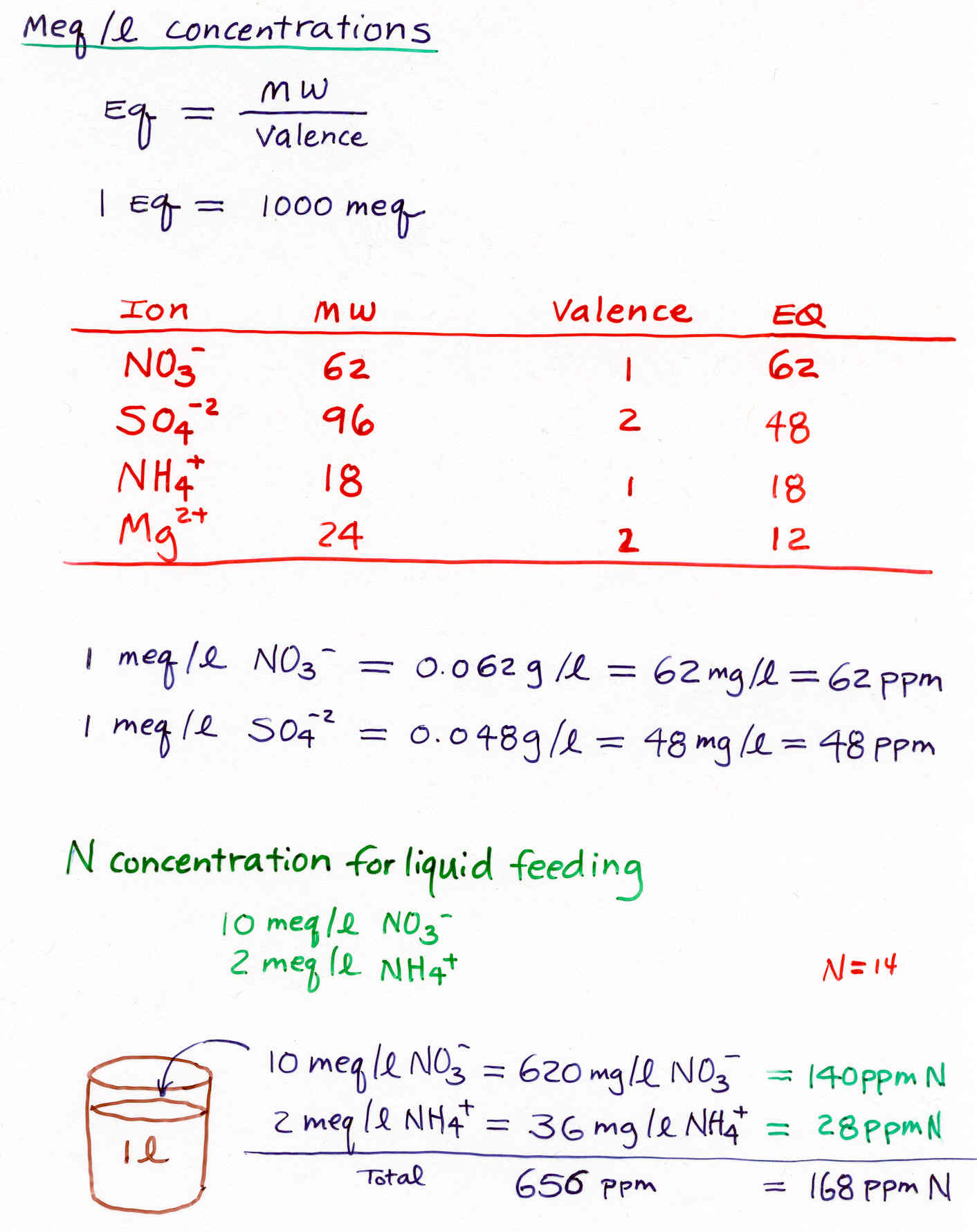
mM = millimolar (1 mM = 0.001 M)
meq/l = milliequivalent per liter (1 meq = 0.001 eq)
V. HYDROPONICS
A. Fertilizers
1)
Hoagland Solution (1950)
2) Modifications of Hoagland solution
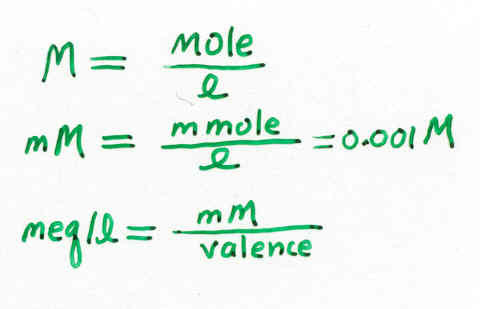
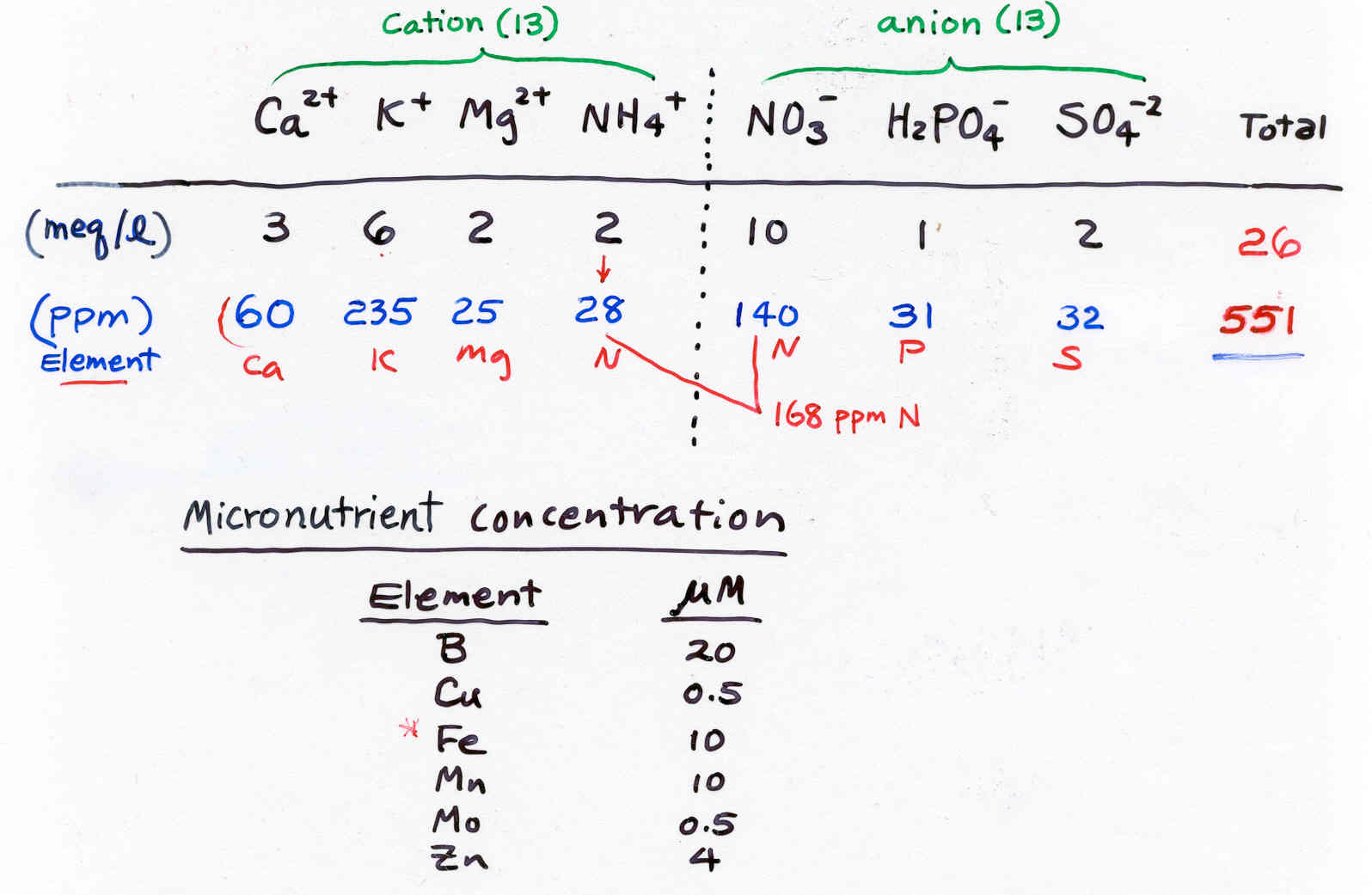
Major Nutrients (Ca, Mg, K, N, P, S)
B. Systems
1) Aeroponics
2) Hydroponics (closed system, open system)
3) Aggregate Culture----Gravel, Sand, Rockwool