A. Temperature Units:
oF,
oC,
Kelvin (K)
|
|
|
|
| Freezing | Boiling | |
|
|
0 | 100 |
|
|
32 | 212 |
F = 9/5 oC + 32B. Heat Units: cal, kcal, BTU
C = 5/9 (oF - 32)
Precise temperature measurements are made by using thermocouples or thermistors
Table 1. Temperature Conversion Table
To convert a temperature in either Celsius or Fahrenheit to the other scale, find that temperature in the center column, and then find the equivalent temperature in the other scale in either the Celsius column to the left or in the Fahrenheit column to the right.
On the Celsius scale the temperature of melting ice is 0oand tile temperature of boiling water is 100oat normal atmospheric pressure. On the Fahrenheit scale the equivalent temperatures are 32o and 212o respectively. The formula for converting Celsius to Fahrenheit is 'F = % oC + 32, and the formula for converting Fahrenheit to Celsius is oC = -5/9(oF - 32).
oC oC or oF oF oC oC or oF oF oC oC or oF oF -73.33 -100 -148.0 -6.67 20 68.0 15.6 60 140.0 -75.56 -95 -139.0 -6.11 21 69.8 16.1 61 141.8 -67.78 -90 -130.0 -5.56 22 71.6 16.7 62 143.6 -65.00 -85 -121.0 -5.00 23 73.4 17.2 63 145.4 -62.22 -80 -112.0 -4.44 24 75.2 17.8 64 147.2 -59.45 -75 -103.0 -3.89 25 77.0 18.3 65 149.0 -56.67 -70 -94.0 -3.33 26 78.8 18.9 66 150.8 -53.89 -65 -85.0 -2.78 27 80.6 19.4 67 152.6 -51.11 -60 -85.0 -2.78 27 80.6 19.4 67 152.6 -48.34 -55 -67.0 -1.67 29 84.2 20.6 69 156.2 -45.56 -50 -58.0 -1.11 30 86.0 21.1 70 158.0 -42.78 -45 -49.0 -0.56 31 8708 21.7 71 15 -40.0 -40 -40.0 0 32 89.6 22.2 72 161.6 -37.23 -35 -31.0 22.8 75 163.4 -34.44 -30 -22.0 0.56 33 91.4 23.3 74 165.2 -31.67 -25 -13.0 1.11 34 93.2 23.9 75 167.0 -28.89 -20 -4.0 16.7 35 95.0 24.4 76 168.8 -26.12 -15 5.0 2.22 36 96.8 25.0 77 170.6 -23.33 -10 14.0 2.78 37 98.6 25.6 78 172.4 -20.56 -5 23.0 3.33 38 100.4 26.4 79 174.2 -17.8 0 32.0 3.89 39 102.2 26.7 80 176.0 4.44 40 104.0 27.2 81 177.8 -17.2 1 33.8 5.00 41 105.8 27.8 82 179.6 -16.7 2 35.6 5.56 42 108.6 28.3 83 181.4 -16.1 3 37.4 6.11 43 109.4 28.9 84 183.2 -15.6 4 39.2 6.67 44 111.2 29.4 85 185.0 -15.0 5 41.0 7.22 45 113.0 30.0 86 186.8 -14.4 6 42.87 7.78 46 114.8 30.6 87 188.6 -13.9 7 44.6 8.33 47 116.6 31.1 88 190.4 -13.3 8 46.4 8.89 48 118.4 31.7 89 192.2 -12.8 9 48.2 9.44 49 120.2 32.2 90 194.0 -12.2 10 50.0 10.0 50 122.0 32.8 91 195.8 -11.7 11 51.8 10.6 51 123.8 33.3 92 197.6 -11.1 12 53.6 11.1 52 125.6 33.9 93 199.4 -10.6 13 55.4 11.7 53 127.4 34.4 94 201.2 -10.0 14 57.2 12.2 54 129.2 35.0 95 203.0 -9.44 15 59.0 12.8 55 131.0 35.6 96 204.8 -8.89 16 60.8 13.3 56 132.8 36.1 97 206.6 -8.33 17 62.6 13.9 57 134.6 36.7 98 208.4 -7.78 18 64.4 14.4 58 136.4 3702 99 210.2
a. cal = calorie
= Amount of heat required to raise the temp of 1g water by 1C (specifically
from 15 oC to 16 oC)
(1 kcal = 1000 cal)
b. BTU = British Thermal Unit
= Amount of heat required to raise the temp of 1 lb water by 1oF
(specifically from 62 F to 63F)
(1 BTU = 252 cal)
C. Heat Absorption and Release by Water
Solid heat
> Liquid
heat*
> Gas
(Ice)
(water)
(vapor)
Vapor -
heat >
Water -
heat** > Ice
*Heat of vaporization = 970
BTU/lb
When water evaporated, heat energy (979
btu/lb) is absorbed from the surrounding.
This phenomenon is utilized in the evaporative cooling system in greenhouse
production.
**Heat of Fusion
When water freezes, it releases heat to the surrounding.
This phenomenon is widely applied in horticulture
- Sprinking of water on the trees to form ice
in a citrus orchard
D. Dry-Bulb and Wet-Bulb Temperatures
2. Movement of HeatEnergy
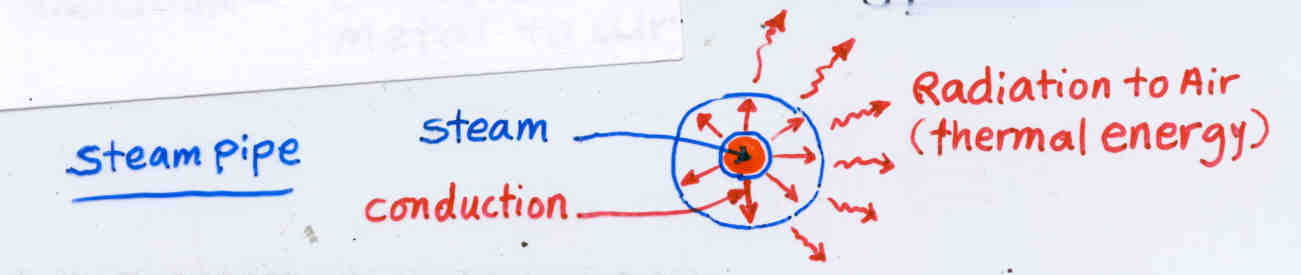
A. Convection - Transfer of heat from one place to another by air or water movement


C. Radiation - Emission of heat energy from a surface of metal to air
3. Biological Effects of Temperature
A.
Q10 - Change in rate of chemical reaction brought about by a
10 oC temperature rise
The rate of reaction doubles every time temp rises 10 oC
- To reduce the rate of respiration, fruits and vegetables are stored in
the refrigerator.
B. High
temperature damage
Desiccation injury due to excessive water loss
Heat tolerance by production of heat-shock
proteins
C. Low
temperature damage
 |
When cell saps (protoplasm) or intercelluar water freeezes, ice crystals are formed. These ice crystals rupture cell walls, making the cell contents leak out and killing the plant. The tissues of cold tolerant plants like evergreens do not form ice crystals, keeping cell walls intact. |
Supercooling- presence of water in
liquid or non-crystalline state below the freezing point by osmotic adjustment
Example - pine needles
D. Hardening
Cold
acclimation - plants acclimate themselves
Cold acclimatization - we acclimatize plants
Increase in the concentration of carbohydrates
(sugars) as osmoticum
E. Cryogenic
Storage
Storage in liquid N (-196 oC, -320
oF)
-
pollen, seed, meristem
4. Dewpoint and Frost
A. Dewpoint
Temperature at which RH reaches 100%
Condensation (dew) of water vapor at temp dew point, 32F
B. Frost
White Frost - Frozen dew occurs when air temp
is dew point 32F and when humidity is high
Black Frost - freezing of tissue appearing
'dark' occurs when humidity is low (at above dew point, below 32F)
C. Causes of Frost
Radiation frost - formed by rapid radiation
of heat to air (clear day, 32F by weather
forecast)
Air-mass freeze - by cold air mass with temp
below 32F (most winter cold damage)
5. Thermal Belt and Temperature Inversion
A. Thermal belt
A relatively warm microclimate formed by cold air drainage, usually on
a slope
good site for orchard
South-facing vs. north-facing
B. Frost pocket
Usually formed by temperature inversion in low areas of a valley site for cold drainage
use wind machine to mix air
6. Temperature Controls
A. Cultural practices
Mulching - use of crop residue or organic materials
use of plastics (clear vs. opaque)
B. Frost control
1) by Escape
- Wait seedling transplanting until it is frost-free (mid
May in Fargo?)
- Delay blooming - variety selection, evaporative
cooling
- Use of INA bacteria? (strawberry in California)
genetically engineered or mutant ice-nucleating active bacteria

2) by Reduction of heat loss
Hotcaps
Plastic tunnels
Cold frames - for seedling culture
3) by addition of heat
Smudging - burning
of fuel in orchard
Water sprinkling
(heat of fusion released) to form ice on plant
Florida orchards
7. Plant Growing Structures
CEA- Controlled Environment Agriculture (Read page
285 text)
(Greenhouse Production)
a. Cold frames - inexpensive, mostly for seedling culture, bulb-forcing
b. Hotbeds -seedling culture
Heat source - composting, electrical, hot water
c. Cloches and plastic tunnels - extensively used in Europe and Japan
d. Greenhouses - more than 85% used for floriculture
(US)
over 65% used for vegetables in Europe
e. Shade houses - for hardening plants for transplanting
(mostly for nursery crops)