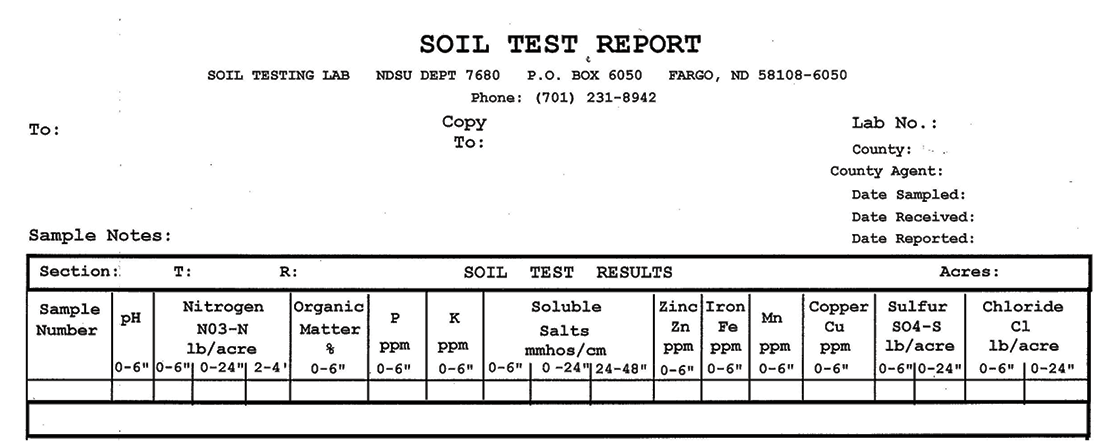
The first chemical analysis category listed on the soil test report is soil pH (Figure 3). The pH scale ranges from 0 to 14 and indicates the level of hydrogen ions in the soil solution. Active hydrogen ions (H+) cause soil acidity, while hydroxyl groups (-OH) cause soil alkalinity.
A soil that contains a high amount of hydrogen ions is called “acidic” (pH below 7) and a soil that contains a high amount of hydroxyl ions is referred to as “basic” or “alkaline” (pH above 7). A soil with a pH of 7 is referred to as “neutral.”
The ideal soil pH for managing most turfgrasses is 6.5 because most nutrients are available at this slightly acidic level. However, various grass species may differ in their tolerance for different pH levels. Maintaining the proper pH is important because some vital nutrients become “fixed” (held tightly) by the soil and become unavailable to the turf as the soil pH extends above or below neutral.
Although a pH of 6.5 is optimal, turfgrasses will grow and, in most cases, thrive in soils above or below that ideal benchmark as long as proper cultural practices are used.
Soils in North Dakota tend to be alkaline and range as high as 8.5 to 9. At these high pH levels, turf yellowing caused by iron chlorosis is quite common because iron molecules in the soil become unavailable to turf roots. As a result, chlorophyll production in plant cells is hindered and the leaves begin to turn dull green to yellow. See the section on “Iron” to correct iron chlorosis of turf.
Soil pH is based on a logarithmic scale. Therefore, a soil with a pH of 8.0 is 10 times more alkaline than a soil with a pH of 7.0 and a soil with a pH of 9.0 is 100 times more alkaline than a soil with a pH of 7.0.
Turf managers can attempt to adjust the soil pH downward (more acidic) by incorporating a sulfur amendment into the soil.
However, the soil has the ability to withstand chemical change and the sulfur amendment may not be effective if the soil pH is high, and if large amounts of calcium carbonate are present. If the soil test lists a pH of 7.5 or higher, a follow-up calcium carbonate test can be conducted at home using a simple process. Place 2 tablespoons of household vinegar in a one-cup disposable container. Then add approximately 1 tablespoon of soil. As the soil absorbs the vinegar, listen for a fizzing sound. If the mixture fizzes, then calcium carbonate is present in high quantities and a sulfur amendment would not be advised.
If the vinegar-soil mixture does not fizz, then a sulfur amendment can be incorporated into the high pH soil. Refer to Table 1 for directions in using sulfur to acidify the soil.
Table 1. Approximate amounts of elemental sulfur needed to lower pH for various soil textures.
|
Change in pH desired
|
Pounds sulfur/1,000 sq. ft.
|
|
Sand
|
Loam
|
Clay
|
|
Do not attempt to add sulfur to lower the soil pH if high amounts of calcium carbonate are present. In addition, irrigation water containing high levels of dissolved carbonates can also raise the soil pH and nullify the effects of a sulfur soil amendment.
|
|
8.5 to 6.5
|
46
|
57
|
69
|
|
8.0 to 6.5
|
28
|
34
|
46
|
|
7.5 to 6.5
|
11
|
18
|
23
|
|
7.0 to 6.5
|
2
|
4
|
7
|
Before planting a new lawn, incorporate the sulfur amendment into soil to a depth of 6 inches before planting. For established turfgrasses, reduce the rate by half and apply only during spring or fall in conjunction with core aeration, and do not apply more than 5 pounds per 1,000 square feet at any one time.
If applying elemental sulfur to bentgrass putting greens, do not apply more than 2 pounds per 1,000 square feet at any one time. If applying to annual bluegrass putting greens, do not apply more than 0.8 pound per 1,000 square feet at any one time. (Fagerness et al., 1998).
Soil pH can also be adjusted upward with lime if the soil is acidic. Because soils in North Dakota are predominantly alkaline, the necessity to lime the soil to adjust the pH upward is extremely uncommon. Do not use lime to treat dog urine damage in lawns.