Maternal amino acid supplementation from pre-breeding through early gestation alters fetal muscle development
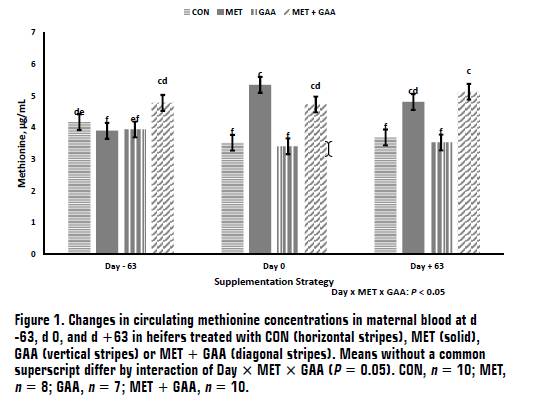
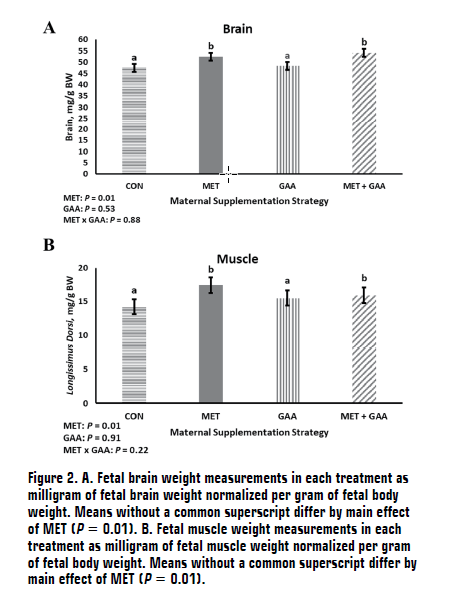
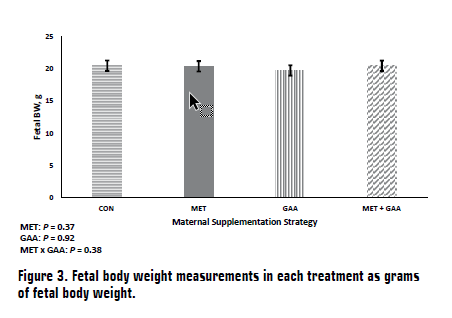
(AS2100-4, December 2023)The objectives of this study were to evaluate effects of specific amino acid supplementation during pre-breeding through early gestation on subsequent blood amino acid concentrations in the heifer and their effects on early fetal development. Methionine and guanidinoacetic acid are amino acids that can impact key metabolic processes called one-carbon metabolism. Proper one-carbon metabolism is essential for fetal development and postnatal outcomes, including muscle growth. In this study, supplementing methionine during breeding and early gestation resulted in increased fetal muscle size; however, supplementation of guanidinoacetic acid did not impact fetal growth.