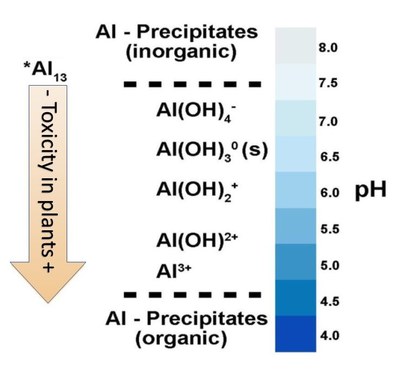
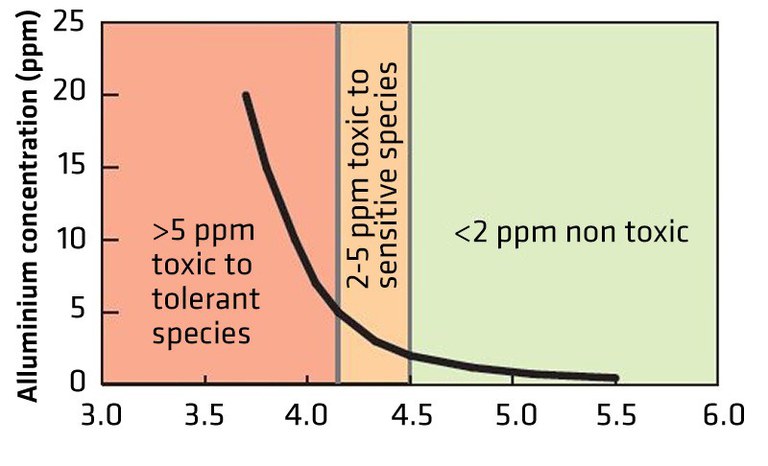
Bojorquez-Quintal, E.B., C.E. Magaña, I.E. Machado and M.M. Estévez. 2017. Aluminum, a friend or foe of higher plants in acid soils. Front. Plant Sci. 8:1767. doi:10.3389/fpls.2017.01767. 18p.
Chien, S.H., R.L. Kallenbach and M.M. Gearhart. 2010. Liming requirement for nitrogen fertilizer-induced soil acidity: A new examination of AOAC Guidelines. Better Crops, 94(2):8-10.
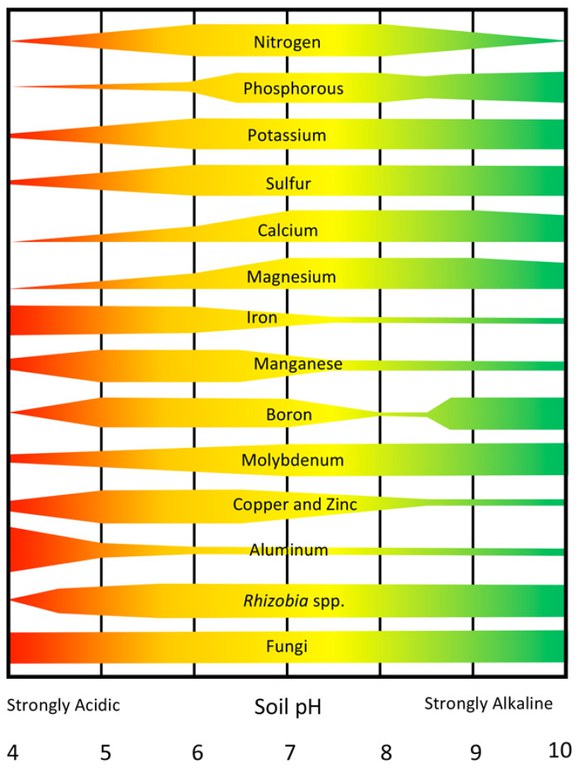
Gazey, P. 2004? Soil acidity. https://soilquality.org.au. (Accessed Dec. 14, 2020).
Havilin, J.L., J.D. Beaton, S.L. Tindall and W.L. Nelson. 2005. Soil fertility and fertilizers, 7th ed. Pearson-Prentice Hall. p. 54.
Kissel, D.E., B.R. Bock and C.Z. Ogles. 2020. Thoughts on acidification of soils by nitrogen and sulfur fertilizers. Agrosyst. Geosci. Environ. 2020, 3:e20060. doi: 10.1002/agg2.2000.
Mamo, M., C. Wortmann and C. Shapiro. 2003. Lime use for soil acidity management. NebGuide G1504. Univ. of Nebr. Ext., Lincoln, Neb.
Neenu, S., and K.S. Karthika. 2019. Aluminum toxicity in soils and plants. Harit Dhara 2(1):15-19.
Wortman, C.S., M. Mamo and C.A. Shapiro. 2015. Management strategies to reduce
the rate of soil acidification. Univ. of Nebr. Ext. NebGuide G1503. Lincoln, Neb. June 2015. 4p.