Early blight foliar lesions can be diagnosed in the field by the characteristic dark concentric rings alternating with bands of light-tan tissue, giving them a distinctive target spot appearance (Figure 1a). Initial symptoms of early blight appear as small, circular or irregular dark brown to black spots, typically starting on the older (lower) leaves (Figure 1b). These spots enlarge up to 3/8 inch in diameter and gradually may become angular in shape, often restricted by leaf veins as they expand.
Early blight foliar lesions develop characteristic dark concentric rings, giving a distinctive target spot appearance.
Lesions initially appear as small, circular to irregular dark brown spots on older (lower) leaves.
Initial early blight lesions may be confused with brown spot (caused by small-spore Alternaria species) (Figure 2a) or black dot (caused by Colletotrichum coccodes) lesions (Figure 2b). However, brown spot and black dot lesions do not develop dark concentric rings characteristic of early blight infection. Brown spot lesions rarely become larger than 3-4mm and are typically round. First early blight lesions appear about two to three days after infection, with sporulation at the lesion margin occurring three to five days later.
Initial early blight lesions may be easily confused with brown spot lesions, caused by small-spored Alternaria species
or black dot caused by Colletotrichum coccodes.
Multiple lesions on the same leaf may coalesce, or grow together, to form what appears to be one large lesion (Figure 3a), potentially resembling grey mold caused by Botrytis cinerea (Figure 3b). Chlorosis (yellowing of plant tissue) may be visible around clusters of infections by the early blight pathogen (Figure 3a). Elongated, brown to black lesions may develop on the stems and petioles of infected plants (Figure 4).
Chlorotic symptoms may develop as early blight lesions coalesce and form clusters.
Or what appears as a large lesion, potentially resembling grey mold caused by Botrytis cinerea.
Elongated, brown to black lesions may develop on the stems and petioles of infected plants.
Later in the growing season, as infections become more severe, lower leaves may drop, and numerous lesions may appear on the upper leaves (Figure 5).
As early blight progresses during the season, numerous lesions may appear on younger leaves in the upper canopy and leaves may drop or dehisce from the stem as infection becomes severe.
Premature leaf senescence, reduced yield and low tuber dry matter content are likely when plants suffer from severe foliar infection during the tuber bulking stage.
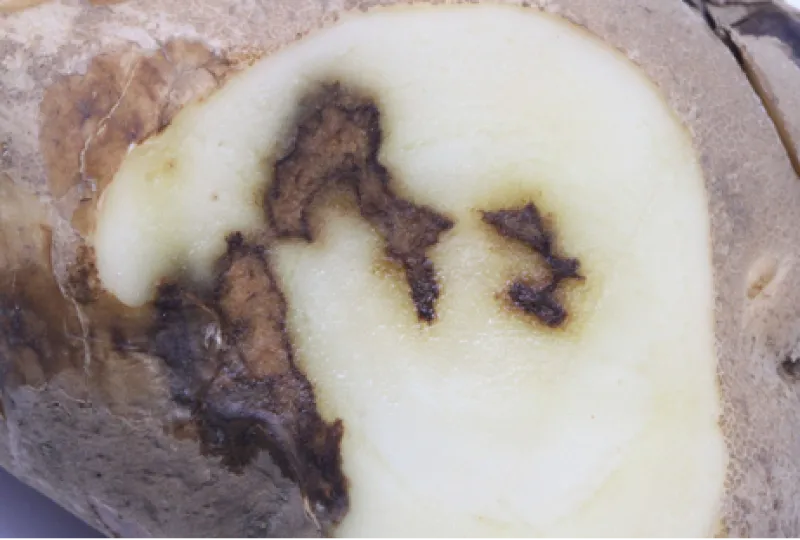
Early blight tuber symptoms appear as dark and sunken lesions on the surface (Figure 6). Tuber lesions may be circular or irregular in shape and surrounded by a raised dark brown border (Figure 7).
Early blight symptoms on infected tubers appear as sunken, dark-colored lesions on the tuber surface.
Early blight tuber lesions may be circular or irregular in shape and often are accompanied by a large, raised, dark brown border.
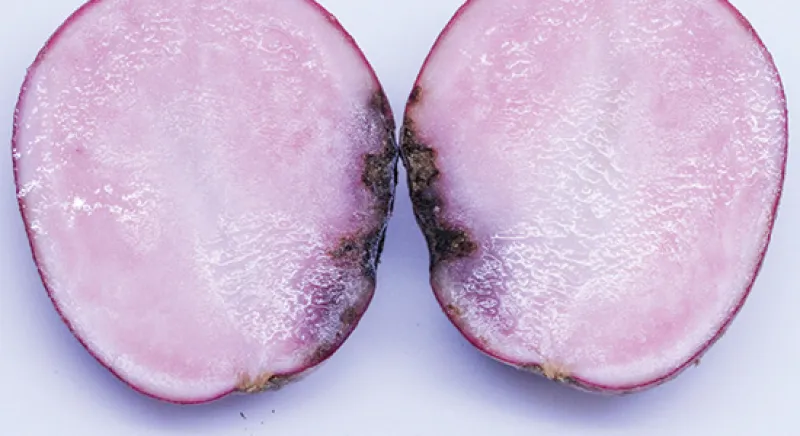
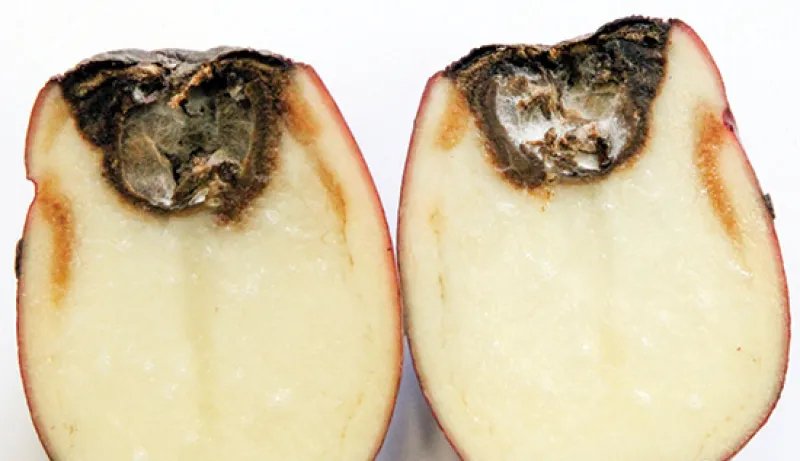
The underlying tuber tissue is dry and dark brown with a corky texture (Figure 8). Tuber symptoms of early blight may manifest only after months of storage (Figure 9) and can be confused with Fusarium dry rot (Figure 10).
Underlying tissues of early blight tuber lesions are usually dry with a corky texture and dark brown color.
In storage, early blight tuber lesions may continue to develop, but secondary spread of infection to non-infected tubers does not occur.
Fusarium dry rot, as illustrated above, can be confused easily with early blight. However, Fusarium dry rot may have more extensive rotting that can lead to tuber tissues collapsing or shrinking, causing internal cavities accompanied by a yellow, pink or white mold.