Plant diseases can be a serious problem in canola production. Rotations must be planned carefully to keep disease incidence and levels low. The major diseases of importance are sclerotinia wilt, blackleg, and clubroot. The diseases of canola less often reported are white rust or staghead, downy mildew, alternaria blackspot and aster yellows.
The blackleg disease occurs in two strains: a mild strain and a virulent (severe) strain. The virulent strain produces deep-stem girdling cankers near the soil line. These cankers reduce plant vigor and may cause lodging. The virulent strain of blackleg first was found in North Dakota during the 1991 growing season.
The blackleg fungus is spread by rain-splashed or wind-borne spores and infected seed. Hybrids that have good tolerance or resistance to this disease are available. As new varieties and hybrids are introduced, more will be resistant to this disease.
In areas where the virulent strain of blackleg is present, crop rotation and selection of resistant varieties is important to blackleg management. Several fungicides are labeled for control of blackleg and should be applied at the two- to four-leaf stage of canola. For detailed information, see NDSU Extension publication PP622 North Dakota Field Crop Plant Disease Management Guide.
Blackleg is not a problem in mustards (yellow, brown and oriental), which are highly resistant. See NDSU Extension publication PP1988 Canola Diseases: Blackleg for more information.
Sclerotinia stem rot or white mold is a canola disease that can be very destructive during periods of wet weather. The sclerotinia fungus survives up to five or six years in the soil in the form of hard, black fungus bodies called sclerotia. Whenever wet weather occurs for a week or two, with moist soil, the sclerotia germinate to produce tiny mushroomlike bodies called apothecia. These apothecia are only 1/8 to 3/16 inch across, yet they produce millions of air-borne spores. Canola primarily is susceptible during all bloom stages and shortly thereafter. The spores infect the dead canola blossom tissue during periods of wet weather.
Infections that start on the dead blossoms spread to adjacent tissues, resulting in dead branches or plants, causing the plants to lodge. The rotted stems usually have a bleached appearance. Sclerotinia infections can be serious on canola if cool, wet weather occurs in the last two weeks of June and continues into early July, when blossoming occurs.
A minimum of a three-year rotation is recommended for fields that have a history of heavy sclerotinia or white mold infestations. During this rotation, avoid planting highly susceptible crops, including sunflower and dry bean.
Various fungicides are registered for use in the suppression and control of sclerotinia in canola. Information on fungicides registered is available in the NDSU Extension publication PP622, North Dakota Field Crop Plant Disease Management Guide.
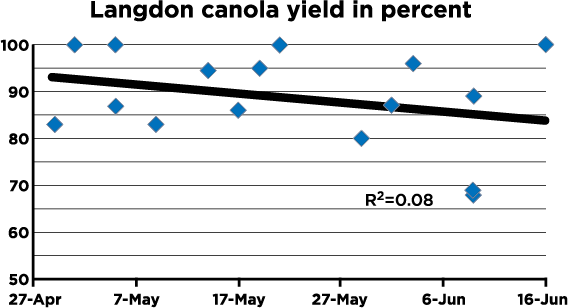
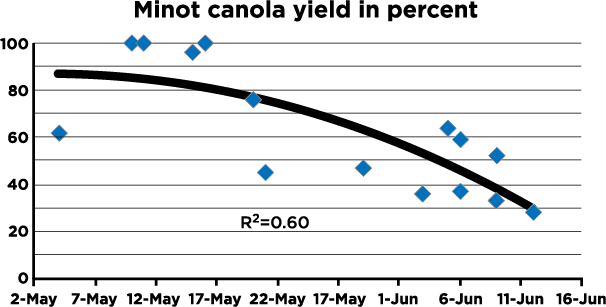
The decision to spray should be made only when: 1) yield potential is above normal (at least 40 bushels or 2,000 lb./A), 2) weather leading to early bloom has been wet (at least 1 to 2 inches of rain in the two weeks prior to early bloom), 3) more rain or high humidity is expected and 4) sclerotinia has been a problem in recent years in fields currently planted to canola or in other fields nearby. A fungicide is more likely to be needed if canola is planted on tight rotations (three years or less). The NDSU canola pathology program provides a sclerotinia risk map for North Dakota during the growing season.
Clubroot is a serious disease threat to canola production in the U.S. and Canada. In 2013, the disease first was identified in Cavalier County, located in the upper northeastern part of North Dakota. Since 2013, other counties are being monitored for detection of clubroot in North Dakota. Once established, no economical ways to eliminate the pathogen from the soil exist. Early detection of the disease and use of available management tools are critical to slow the spread and limit yield loss from clubroot. For a description of the disease and control see NDSU Extension publication PP1998, Canola Diseases: Clubroot.